| Product No. | NS039 |
|---|---|
| CAS Reg. No. | 198470-84-7 |
| Alternate CAS Reg. No. | - |
| Offer | 50 mg, 100 mg |
198470-84-7
- Documentation
- Details
Chemical name
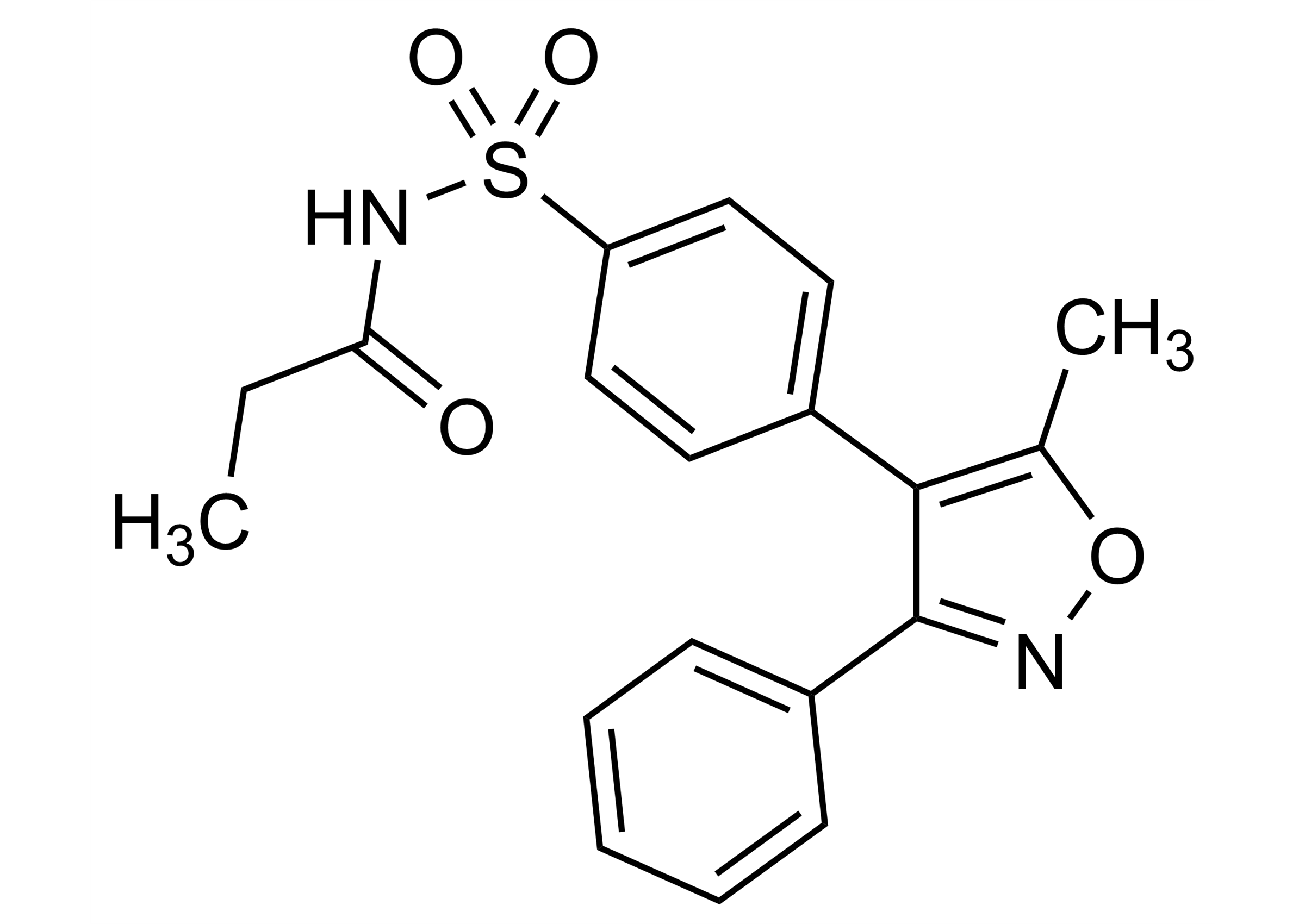
4-(5-Methyl-3-phenyl-isoxazol-4-yl)-N-propionyl-benzenesulfonamide
Description
Parecoxib (CAS 198470-84-7) reference standard enables reliable LC-MS/MS and GC-MS quantification from the very first injection. This COX-2 selective analgesic reference material is produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH. It provides full traceability for calibration, supports method validation, and strengthens confirmatory analysis. Laboratories gain consistent identity and purity, so results remain comparable across instruments, sites, and time. Therefore, this material helps reduce troubleshooting, speeds routine workflows, and safeguards data integrity in regulated environments.
Designed for broad analytical use, this reference standard integrates smoothly into diverse matrices and workflows. Typical applications include:
- Regulated laboratories requiring traceable calibration and system suitability
- Pharmaceutical research supporting pharmacokinetic and toxicological investigations
- Residue control with targeted quantification and stringent reporting limits
- Metabolism studies tracking biotransformation and degradation pathways
- Multi-residue method development with robust selectivity and sensitivity
- GC-MS and LC-MS/MS confirmatory analysis to verify screening findings
Each batch of this reference standard is characterized to confirm identity and purity, ensuring dependable response factors across platforms. Use it to establish calibration curves, verify linearity, assess accuracy and precision, and demonstrate measurement traceability. Clear documentation, including a certificate of analysis, supports technical audits and routine quality assurance. Moreover, consistent lot-to-lot performance helps maintain continuity in long-term studies.
Adopt best practices for handling, storage, and dilution to preserve stability and minimize uncertainty. Prepare fresh working solutions as needed, and verify performance during sequence runs with quality control levels. With its proven reliability, the Parecoxib (CAS 198470-84-7) reference standard from WITEGA Laboratorien Berlin-Adlershof GmbH strengthens validation packages, enhances confirmatory analysis, and streamlines traceable quantification in complex matrices.
Safety Data Sheet
You can download your Safety Data Sheet for NS039
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | 4-(5-Methyl-3-phenyl-isoxazol-4-yl)-N-propionyl-benzenesulfonamide |
|---|---|
| Molecular Formula | C19H18N2O4S |
| Molecular Weight | 370.43 g/mol |
| Isotopic purity | - |
| HPLC purity | > 99.0 % |
| Overall purity | > 99.0 % (HPLC) |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | refrigerator, 2-8°C |
| Country of Origin | Germany |
| Product No. | NS039 |
| CAS Reg. No. | 198470-84-7 |
| Alternate CAS Reg. No. | – |
| Offer | 50 mg, 100 mg |